Experience our innovative solution for life sciences
Unlock the power of Glooko for your clinical trials
From clinical trials to real-world studies, Glooko partners with life sciences organizations for end-to-end clinical evidence development.
With Glooko as your strategic partner, life sciences organizations can bring diabetes treatments and technologies to market faster, more efficiently, and with greater confidence.
Benefits of partnering with Glooko:
- Increase usage of connected products in decentralized and hybrid clinical trials due to demand for remote data collection and virtual visits
- Leverage Glooko’s 21 CFR Part 11 compliant clinical trial platform for study data acquisition
- Remotely capture granular real-world data in nearly real time
- Easily manage multiple diabetes and health monitoring devices used in clinical trials
- Simplify protocols for patients and increase adherence rates
- Efficiently screen and recruit patients
- Data tokenization to unlock a richer, longitudinal view of patient journeys without compromising individual privacy

Achieve success at every step
of a clinical trial with Glooko
Our real-world data capabilities along with our comprehensive trial and data management platforms empowers life sciences organizations to make faster, scalable decisions across the clinical development lifecycle
Protocol Design
Use Glooko’s real-world data from more than 8,000 clinics to help select devices and determine protocol feasibility
Clinical Trial Site Selection & Patient Recruitment
Our real-world data and recruitment tools reveal where your target populations are and how they behave, enabling optimized site selection and precise patient identification to accelerate enrollment and improve clinical trial success.
Data Collection
Continuously collect data remotely in near real time and share with study sponsor throughout trials when patients can’t visit a clinic
Patient Monitoring & Study Management
Remotely monitor patients to ensure they are on track and view site performance via easy-to-use and nearly real-time dashboards
Data Export
Easily export data in a variety of formats for analysis
Protocol Design
Use Glooko’s real-world data from more than 8,000 clinics to help select devices and determine protocol feasibility
Clinical Trial Site Selection & Patient Recruitment
Our real-world data and recruitment tools reveal where your target populations are and how they behave, enabling optimized site selection and precise patient identification to accelerate enrollment and improve clinical trial success.
Data Collection
Continuously collect data remotely in near real time and share with study sponsor throughout trials when patients can’t visit a clinic
Patient Monitoring & Study Management
Remotely monitor patients to ensure they are on track and view site performance via easy-to-use and nearly real-time dashboards
Data Export
Easily export data in a variety of formats for analysis
Unmatched source for
real-world data
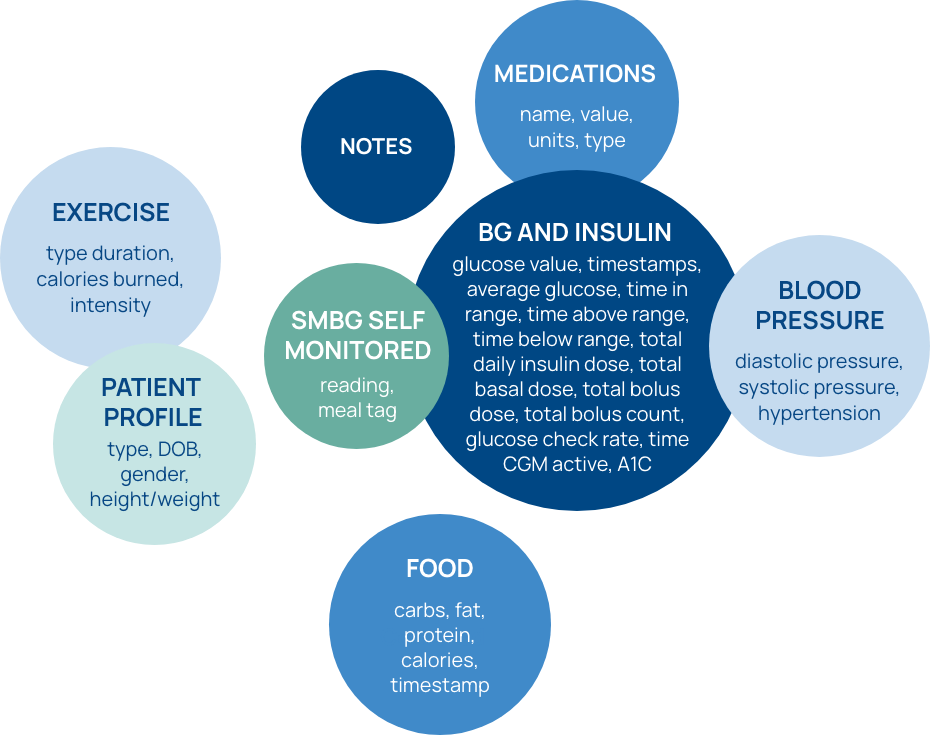
- From acquisition to viewing, visualization, and export, Glooko is an unmatched source for real-world data from patients living with diabetes around the world.
- 100B+ health data points from 30 countries and 20 languages
- Robust datasets available, including glucose, insulin, exercise, food, and medications
- Support for more than 200 diabetes and health monitoring, including BGMs, CGMs, insulin pens, smart scales, and fitness trackers, supported devices with provisioning available
- Real-time data sharing with sponsors during the study
- Compliant with HIPAA, GDPR, ISO 13485, and 21 CFR Part 11